
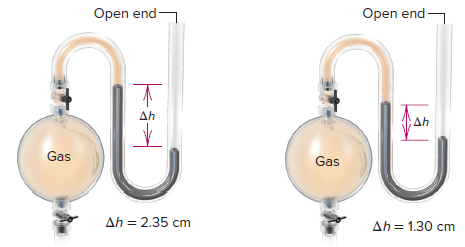
The volume of the gas is 7.80 L, and the pressure is 0.980 atm. Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) The hydrogen gas produced is collected over water at 25.0C. (Vapor pressure of water at 25C = 0.0313 atm.) 5.68 A sample of zinc metal reacts completely with an excess of hydrochloric acid: Calculate the mass of sodium used in the reaction. The volume of the gas is 246 mL measured at 1.00 atm. If the partial pressure of helium is 368 mmHg, what is the partial pressure of neon? (Vapor pressure of water at 28C = 28.3 mmHg.)ĥ.67 A piece of sodium metal reacts completely with water as follows:ĢNa(s) + 2H2O(l ) NaOH(aq) + H2(g) The hydrogen gas generated is collected over water at 25.0C.

5.66 A mixture of helium and neon gases is collected over water at 28.0C and 745 mmHg. Calculate the partial pressures of the gases. 5.63 A mixture of gases contains 0.31 mol CH4, 0.25 mol C2H6, and 0.29 mol C3H8. Deduce from these data the corresponding equation and write formulas for the oxide and sulfate of M. The combustion process isĬH4(g) + 2O2(g) CO2(g) + 2H2O(l) If 15.0 moles of CH4 are reacted, what is the volume of CO2 (in liters) produced at 23.0C and 0.985 atm?ĥ.56 A quantity of 0.225 g of a metal M (molar mass = 27.0 g/mol) liberated 0.303 L of hydrogen gas (measured at 17C and 741 mmHg) from an excess of hydrochloric acid. What is the molecular formula of the gas?ĥ.51 Consider the formation of nitrogen dioxide from nitric oxide and oxygen:ĢNO(g) + O2(g) NO2(g) If 9.0 L of NO are reacted with excess O2 at STP, what is the volume in liters of the NO2 produced? 5.52 Methane, the principal component of natural gas, is used for heating and cooking. At 20C, 0.100 g of the gaseous compound occupies a volume of 22.1 mL and exerts a pressure of 1.02 atm. What is the molecular formula of the compound? 5.50 A compound has the empirical formula SF4.
At 120C and 750 mmHg, 1.00 L of the gaseous compound weighs 2.30 g.

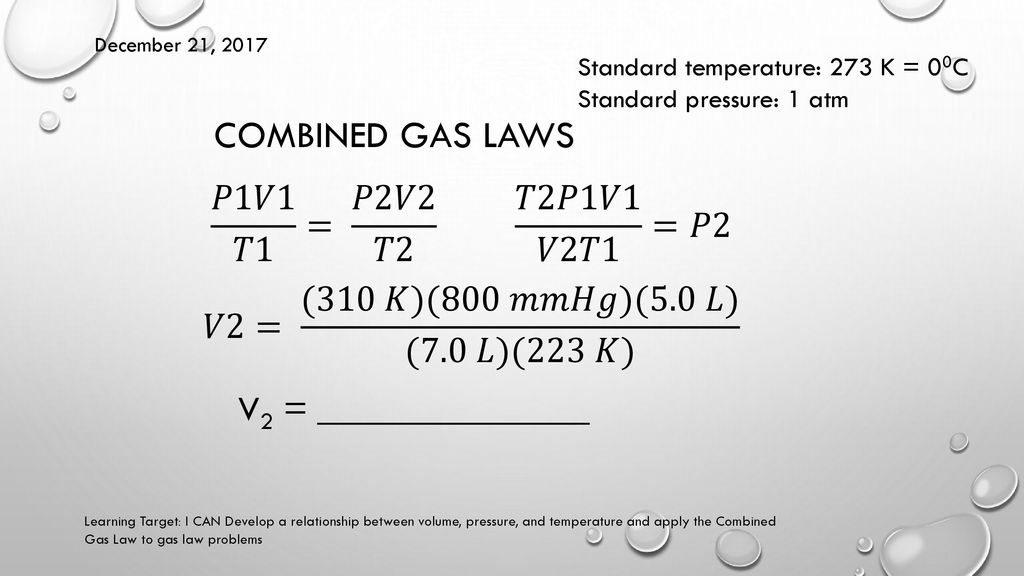
Calculate the molar mass of the gas.ĥ.49 A certain anesthetic contains 64.9 percent C, 13.5 percent H, and 21.6 percent O by mass. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas. A 0.050-g sample of dry ice is placed in an evacuated 4.6-L vessel at 30C. Calculate the number of moles of gas present.ĥ.33 What volume will 5.6 moles of sulfur hexafluoride (SF6) gas occupy if the temperature and pressure of the gas are 128C and 9.4 atm? 5.42 Dry ice is solid carbon dioxide. What is its final temperature? 5.29 What are standard temperature and pressure (STP)? What is the significance of STP in relation to the volume of 1 mole of an ideal gas? 5.31 A sample of nitrogen gas kept in a container of volume 2.3 L and at a temperature of 32C exerts a pressure of 4.7 atm. What is the pressure of the gas in mmHg if the volume is changed to 9.65 L? (The temperature remains constant.)ĥ.24 Under constant-pressure conditions a sample of hydrogen gas initially at 88C and 9.6 L is cooled until its final volume is 3.4 L. What is the pressure when the volume of the gas is reduced to one-tenth (0.10) of the original value at the same temperature? 5.21 The volume of a gas is 5.80 L, measured at 1.00 atm. What is its final volume? 5.20 At 46C a sample of ammonia gas exerts a pressure of 5.3 atm. Which of the following diagrams best represents the situation if the final temperature is (a) above the boiling point of the substance and (b) below the boiling point but above the freezing point of the substance?ĥ.19 A gas occupying a volume of 725 mL at a pressure of 0.970 atm is allowed to expand at constant temperature until its pressure reaches 0.541 atm. Assume that the temperature remains constant.ĥ.17 A gaseous sample of a substance is cooled at constant pressure. What is theĥ.16 Explain why a helium weather balloon expands as it rises in the air. Please visit pressure conversion to convert all pressure units.5.14 The atmospheric pressure at the summit of Mt. To convert atm to bar, multiply the atm value by 1.01325.īar is the atmospheric pressure at the sea level and is equal to 100 kilopascals (kPa).Ītm (atmospheric pressure) is the force per unit area by the weight of air above that point.
#800 mmhg to atm how to#
To convert bar to atm, multiply the bar value by 0.98692326671 or divide by 1.01325.Ītm = bar / 1.01325 How to convert atm to bar?ġ Atm (atmospheric pressure) is equal to 1.01325 bar. How to convert bar to atm (atmospheric pressure)?ġ Bar is equal to 0.98692326671 atmospheric pressure (atm). Alternatively, to find out the atmospheric pressure value for the most commonly converted bar values, you may check the bar to atm table.īelow, you will find information of how to convert bar to atm and how to convert atm to bar, including the formulas and example conversions. To convert bar to atm (atmosphere), you may use the bar to atm converter above.


 0 kommentar(er)
0 kommentar(er)
